What is an electron
An electron is a subatomic particle that carries a negative electric charge. It is one of the fundamental constituents of matter, and it orbits the nucleus of an atom. Electrons are extremely lightweight, with a mass about 1/1836 times that of a proton, which is the other main constituent of an atom's nucleus.
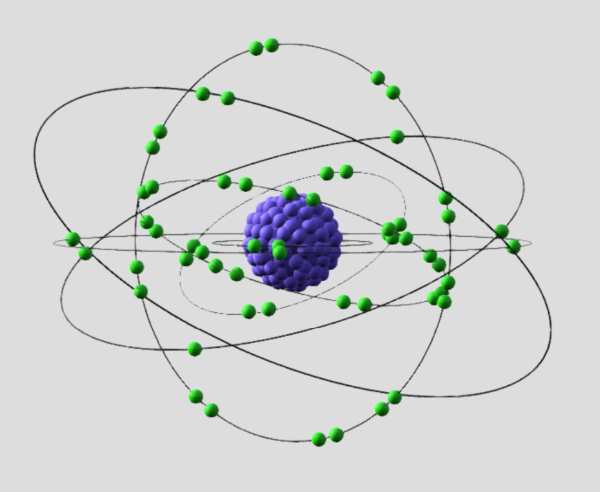
Electrons are often depicted as orbiting the nucleus in discrete energy levels or shells, according to the rules of quantum mechanics. However, modern understanding suggests that electrons are better described as existing in electron clouds or probability distributions around the nucleus, where the probability of finding an electron is highest in certain regions.
Electrons play a crucial role in various physical phenomena, including electricity, magnetism, chemical bonding, and many aspects of modern technology, such as electronics and telecommunications. The behavior of electrons within atoms and in materials forms the basis of much of our understanding of physics and chemistry.
If electrons leave, what will happen to atoms
If electrons were to move away from an atom, the atom would become positively charged. This is because electrons carry a negative charge, and their movement away from the nucleus would leave behind an excess of positive charge. The nucleus, which contains protons and neutrons, would then have a net positive charge.
The loss of electrons from an atom can occur through a process called ionization. Ionization can happen due to various factors, such as exposure to high-energy radiation, chemical reactions, or the application of an electric field. When an atom loses one or more electrons, it becomes an ion.
The behavior of an atom after losing electrons depends on the circumstances. In some cases, it may gain electrons back from its surroundings, especially if it's in an environment where free electrons are available. In other cases, the positively charged ion may interact with other atoms or molecules, leading to chemical reactions or the formation of compounds.